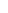TRUMENBA DOSING & SAFETY
Ensure your teen completes all recommended doses of TRUMENBA to help protect them against MenBTRUMENBA follows a 2- or 3-dose schedule1
2-dose schedule for healthy teens
Dose 1
at day 1
Dose 2
6 months after dose 1
ACIP* recommends 2 doses for healthy adolescents and young adults2
ACIP recommends 3 doses (0, 1-2, 6 months) for people 10 years of age or older who are at increased risk2†
ACIP* recommends 3 doses for persons who are at an increased risk of meningococcal disease or in a MenB outbreak situation2
Dose 1
at day 1
Dose 2
1-2 months after dose 1
Dose 3
6 months after dose 1
*The CDC Advisory Committee on Immunization Practices.
†These persons include: persons with persistent complement component deficiencies; persons with anatomic or functional asplenia; microbiologists routinely exposed to isolates of Neisseria meningitidis; persons identified as at increased risk of a MenB disease outbreak.
Discuss with your teen’s health care provider or pharmacist which schedule is appropriate