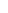TRUMENBA follows a 2- or 3-dose schedule.7 Discuss with your teen’s health care provider or pharmacist which schedule is appropriate for your teen.
ACIP* recommends 2 doses for healthy adolescents and young adults10
ACIP recommends 3 doses (0, 1-2, 6 months) for people 10 years of age or older who are at increased risk10†
Your teen must complete all recommended doses of TRUMENBA to help protect them against MenB. When you're in the office to receive the first dose, make an appointment to receive the next dose so you stay on schedule.
Teens and young adults between ages 16 and 23 may be at higher risk for MenB.11 Ask your teen's health care provider about administering TRUMENBA at 16, when they are receiving a booster dose of a vaccine for other types of meningitis (the MCV4 vaccine, which covers meningitis A, C, W, and Y).7,9
*The CDC Advisory Committee on Immunization Practices.
†These persons include: persons with persistent complement component deficiencies; persons with anatomic or functional asplenia; microbiologists routinely exposed to isolates of Neisseria meningitidis; persons identified as at increased risk of a MenB disease outbreak.