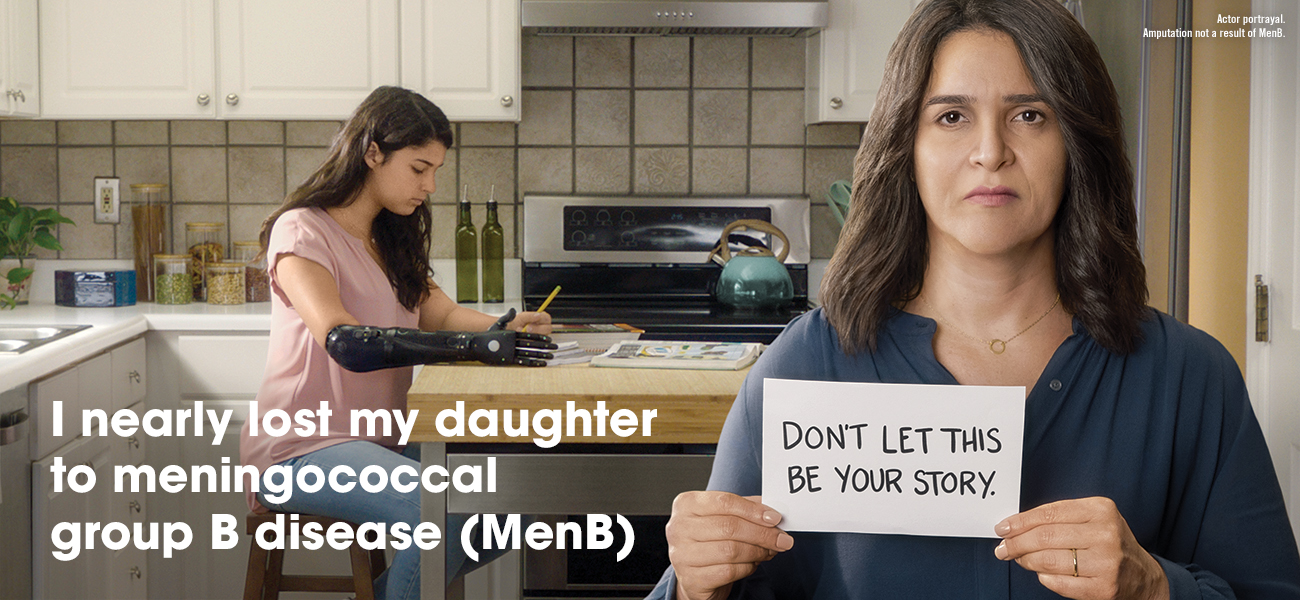
 Use my current location Find it! Oops! Please enter a valid ZIP code
Use my current location Find it! Oops! Please enter a valid ZIP code

Teens and young adults must complete all recommended doses of TRUMENBA to be fully vaccinated against MenB. Get reminders for the next dose
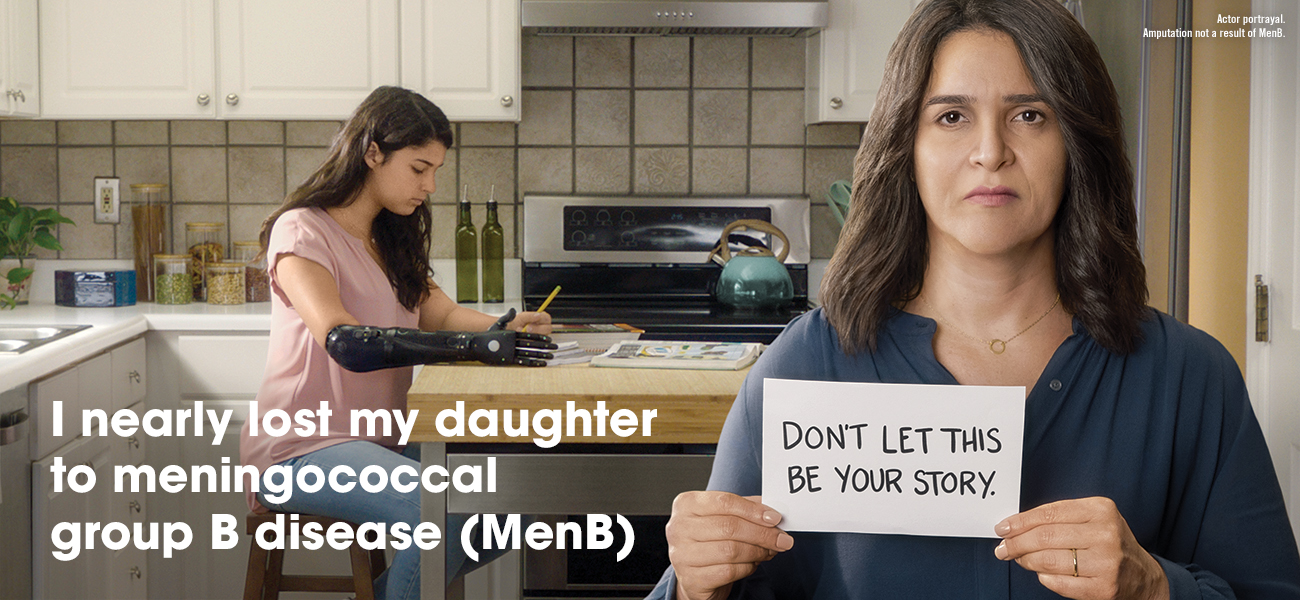
 Use my current location Find it! Oops! Please enter a valid ZIP code
Use my current location Find it! Oops! Please enter a valid ZIP code

MenB is an uncommon but potentially deadly disease that progresses quickly and can lead to death within 24 hours.2,3
There are multiple strains of MenB. TRUMENBA has been shown to help protect teens from the most common strains of MenB.1,4
Teens and young adults between ages 16 and 23 may be at higher risk for MenB. You can help protect your teen by vaccinating with TRUMENBA.1,5 Talk to your health care provider or pharmacist today.
In adolescents and young adults, the incidence of MenB peaks at age 19, so if your child is between 16 and 23, they may be at higher risk.5
TRUMENBA is the only vaccine tested against diverse MenB strains, including those found in meningitis outbreaks.1,4*
Why risk it? MenB is rare but has devastating consequences.3,7 Prep for a doctor's appointment with a Doctor Discussion Guide found at the link below. Then, talk to your doctor about the importance of vaccinating your teen or young adult.
*TRUMENBA was tested against diverse MenB strains expressing factor H binding protein subfamilies A and B. Two-dose effectiveness against diverse strains has not been confirmed.1
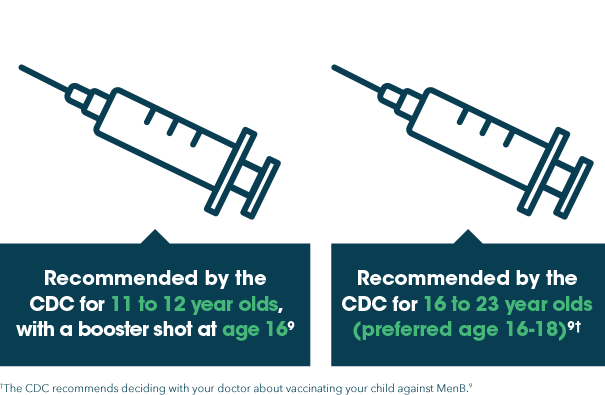
There are 5 primary types of bacteria that cause meningitis (also known as meningococcal disease) and for which vaccines are available in the US: A, C, W, Y, and B.8
The Centers for Disease Control and Prevention (CDC) recommends vaccination for types A, C, W, and Y at ages
You can help protect your teen by vaccinating with TRUMENBA, which is FDA approved for MenB.1
Routine visits with your child's doctor are a good opportunity to discuss the risks and potential serious side effects of MenB.7 Make sure you know whether your teen has received both types of meningitis vaccines, and whether they are protected against MenB.
Sign up for the TRU NextDose reminder program to help ensure your teen or young adult gets all recommended doses.
Get remindersPatients should always ask their doctors for medical advice about adverse events.
You are encouraged to report negative side effects of vaccines to the US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). Visit www.vaers.hhs.gov or call
This site is intended only for U.S. residents. The products discussed in this site may have different product labeling in different countries. The information provided is for educational purposes only and is not intended to replace discussions with a healthcare provider.
